


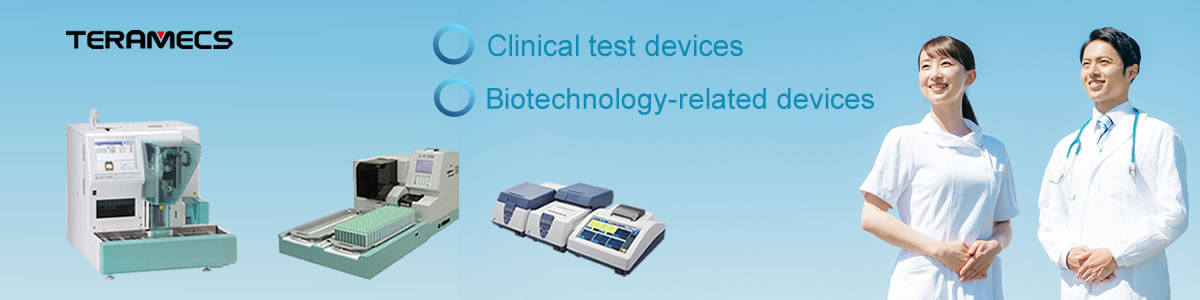
‣Urine analyzers(Semi-and full-automated)
‣Full-automated CLEIA system
‣Full-automated Feces Occult blood analyzer
‣Clinical biochemistry analyzer
Biotechnology-related Devices (LAMP-related devices)

‣Roots are important for growth.
‣Orientation of self-driven management
‣Focus on bio-science
‣Development-led in respect for ingenuity
‣Niche market and state-of-the-art product rather than volume
‣Balance between short-term profit and long-term profit
‣Focus on bio-science
‣Participation in the collaborated researches with academies
‣Incubation, i.e. to grow seedlings in the seed-plot
(Product development and financing)
‣Establishment: February 1986
‣Employee: 53
‣Capital: YEN 40 million
The following certificates were obtained
‣ISO 13485:2016
‣Development, manufacturing, sales and maintenance of clinical test devices
‣Biotechnology-related devices
Address: 354 Nakagawara-chou, Takeda, Fushimi-ku, Kyoto 612-8412, Japan
E-mail:info@teramecs.co.jp
Tel : +81-75-606-2800 Fax: +81-75-606-2770